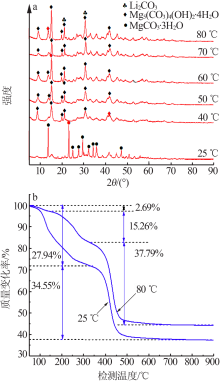
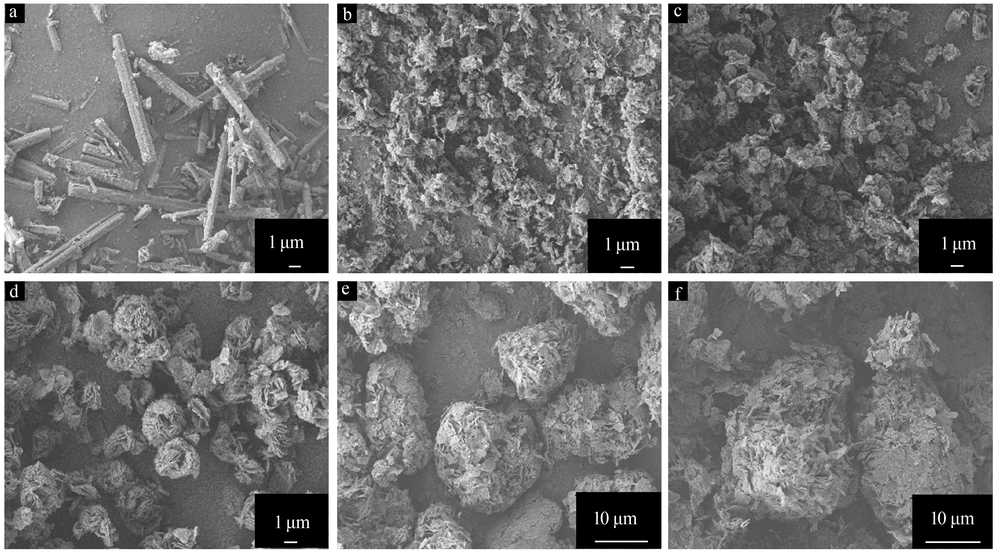
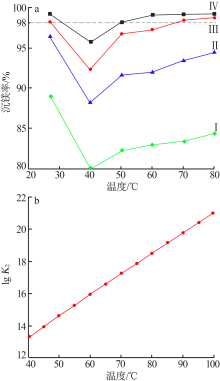
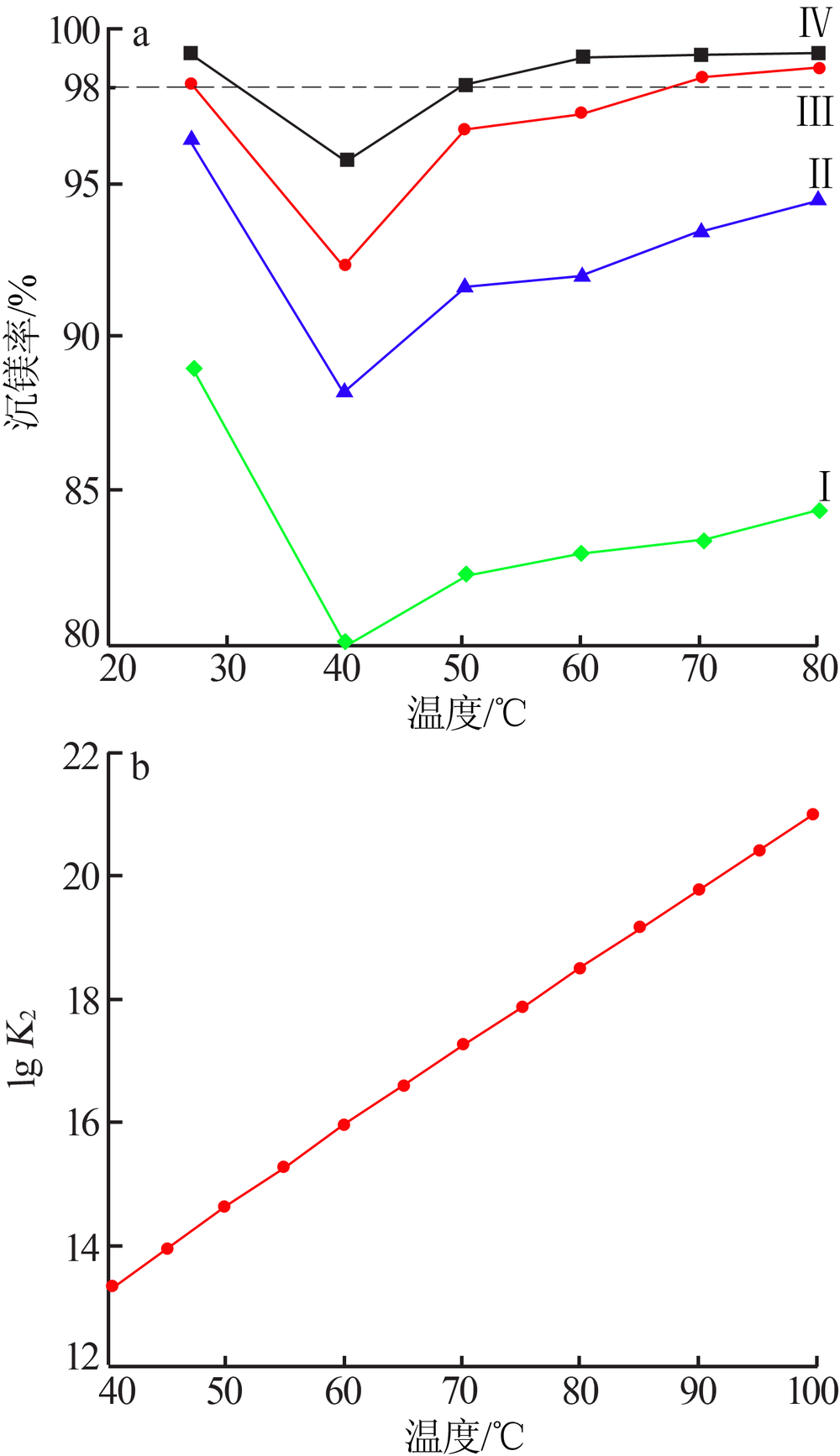
| [1] |
乜贞, 卜令忠, 王云生 , 等. 盐湖卤水资源锂镁分离的工艺技术[J]. 无机盐工业, 2013,45(5):1-4.
|
| [2] |
尹红军, 邓天龙, 李栋婵 . 盐湖卤水资源锂镁分离提取的研究进展[J]. 无机盐工业, 2009,41(5):1-4.
|
| [3] |
付烨, 钟辉 . 沉淀法分离高镁锂比盐湖卤水的研究现状[J]. 矿产综合利用, 2010(2):30-33.
|
| [4] |
李存增, 常华, 柳杰 , 等. 从吸附法盐湖卤水提锂溶液中去除钙、镁试验研究[J]. 湿法冶金, 2014(6):476-479.
|
| [5] |
向兰, 刘峰, 金永成 , 等. 试论我国西部盐湖镁资源的高度利用对策[J]. 盐科学与化工, 2002,31(4):24-27.
|
| [6] |
Cheng W, Li Z B, Demopoulos G P . Effects of temperature on the preparation of magnesium carbonate hydrates by reaction of MgCl2 with NaCO3[J]. Chinese Journal of Chemical Engineering, 2009,17(4):661-666.
doi: 10.1016/S1004-9541(08)60260-8
|
| [7] |
程文婷, 李志宝, 柯家骏 . MgCO3·3H2O晶体生长及晶形的影响因素[C] // 中国有色金属学会.2008年全国湿法冶金学术会议.赣州, 2008: 230-235.
|
| [8] |
Kloprogge J T, Martens W N, Nothdurft L , et al. Low temperature synjournal and characterization of nesquehonite[J]. Journal of Ma-terials Science Letters, 2003,22(11):825-829.
|
| [9] |
付梦源, 程芳琴, 程文婷 , 等. 沉淀结晶法制备不同形貌的碳酸镁水合物[J]. 盐业与化工, 2015,44(12):24-29.
|
| [10] |
Wang Y, Li Z, Demopoulos G P . Controlled precipitation of nespue-honite (MgCO3·3H2O) by the reaction of MgCl2 with (NH4)2CO3[J]. Journal of Crystal Growth, 2008,310(6):1220-1227.
|
| [11] |
邵平平, 李志宝, 密建国 . 碳酸镁水合物在283~363 K范围内的晶体组成及晶型[J]. 过程工程学报, 2009,9(3):520-525.
|
| [12] |
Zhang Z, Zheng Y, Ni Y , et al. Temperature-and pH-dependent mo-rphology and FT-IR analysis of magnesium carbonate hydrates[J]. Journal of Physical Chemistry B, 2006,110(26):12969-12973.
doi: 10.1021/jp061261j
|
| [13] |
杨晨 . 多晶相水合碳酸镁结晶生长过程调控研究[D]. 上海:华东理工大学, 2013.
|
 ),Xiang Lan2(
),Xiang Lan2( )
)