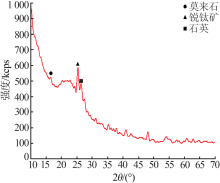
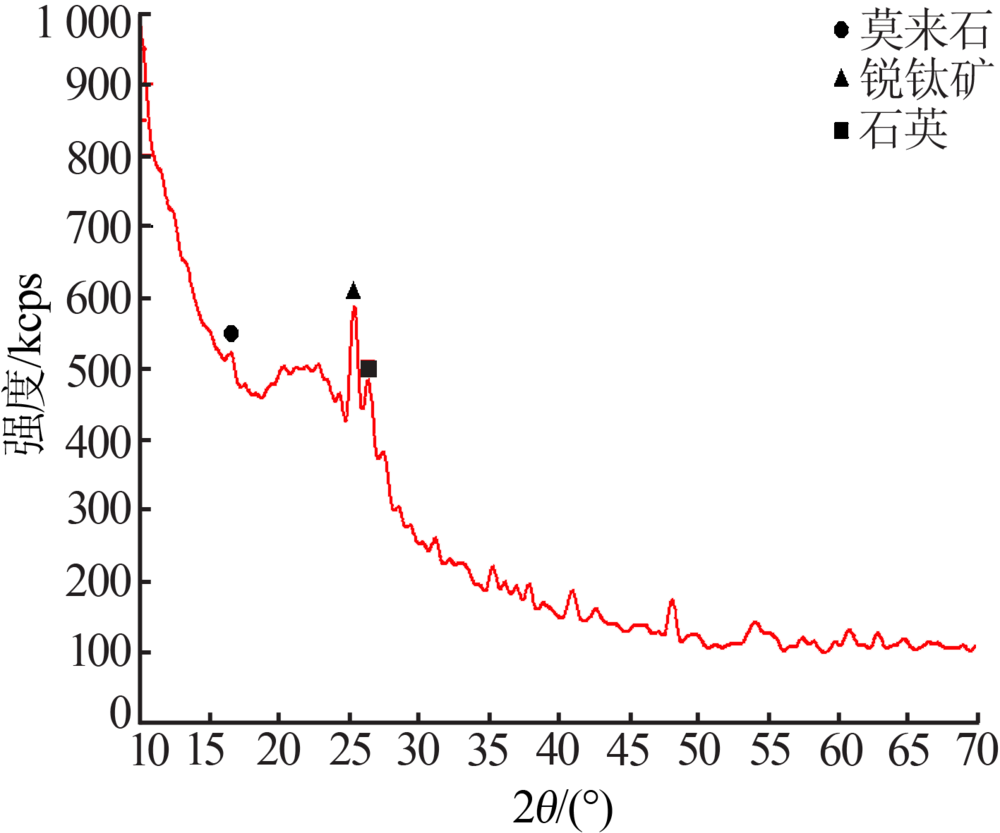
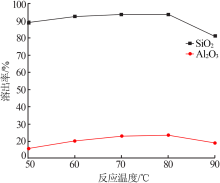
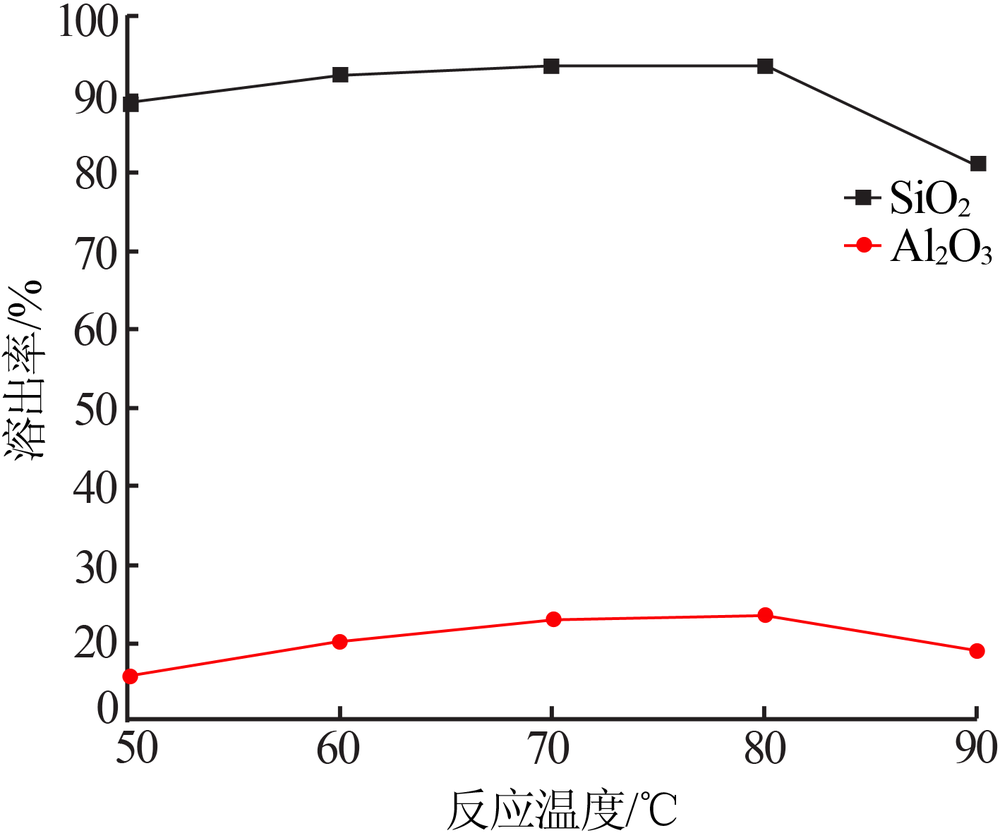
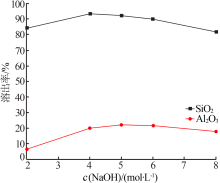
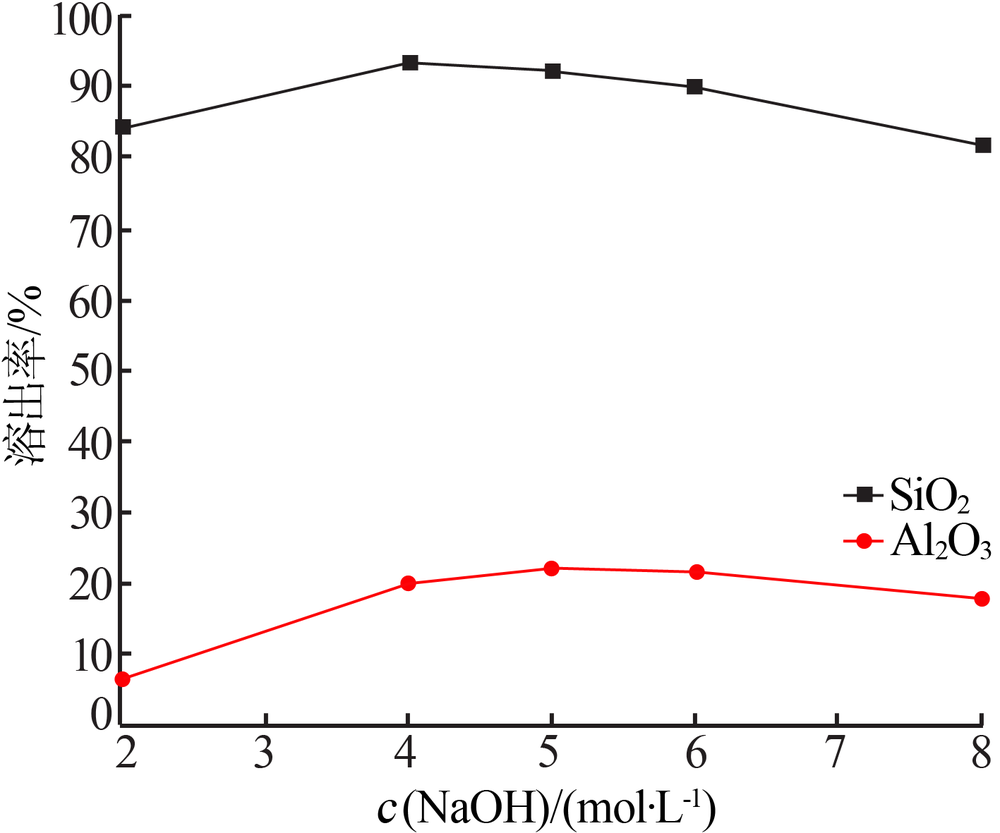
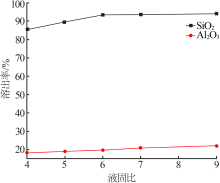
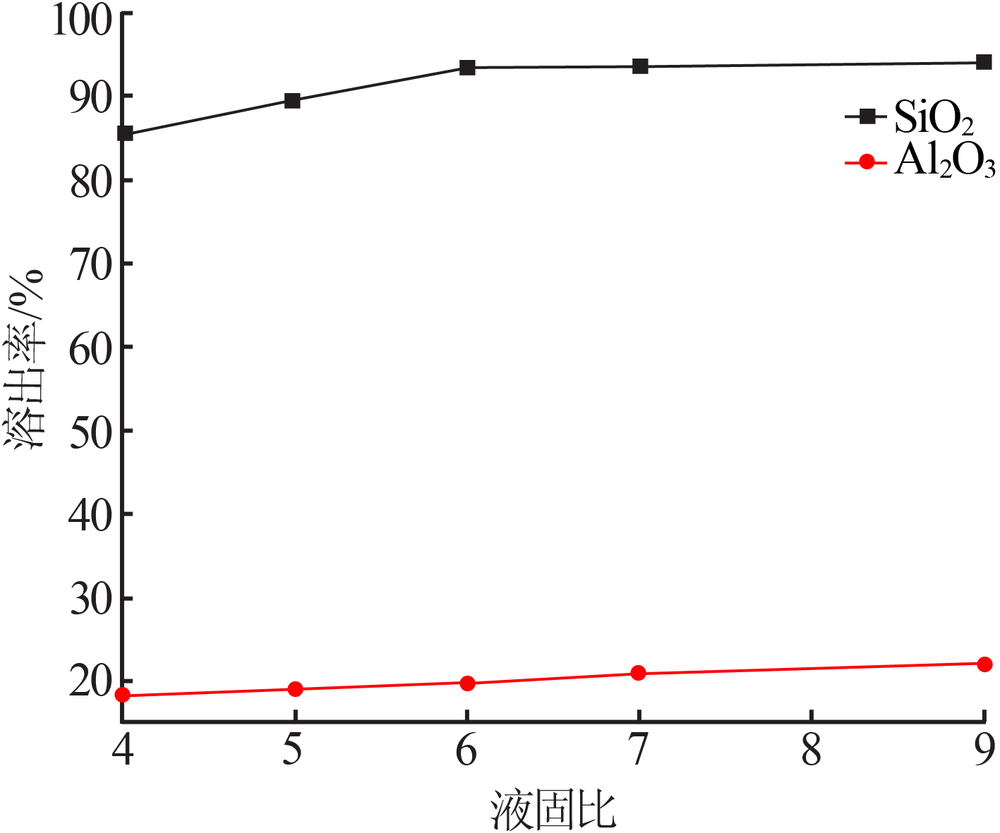
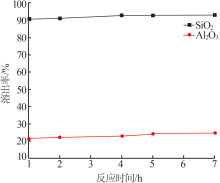
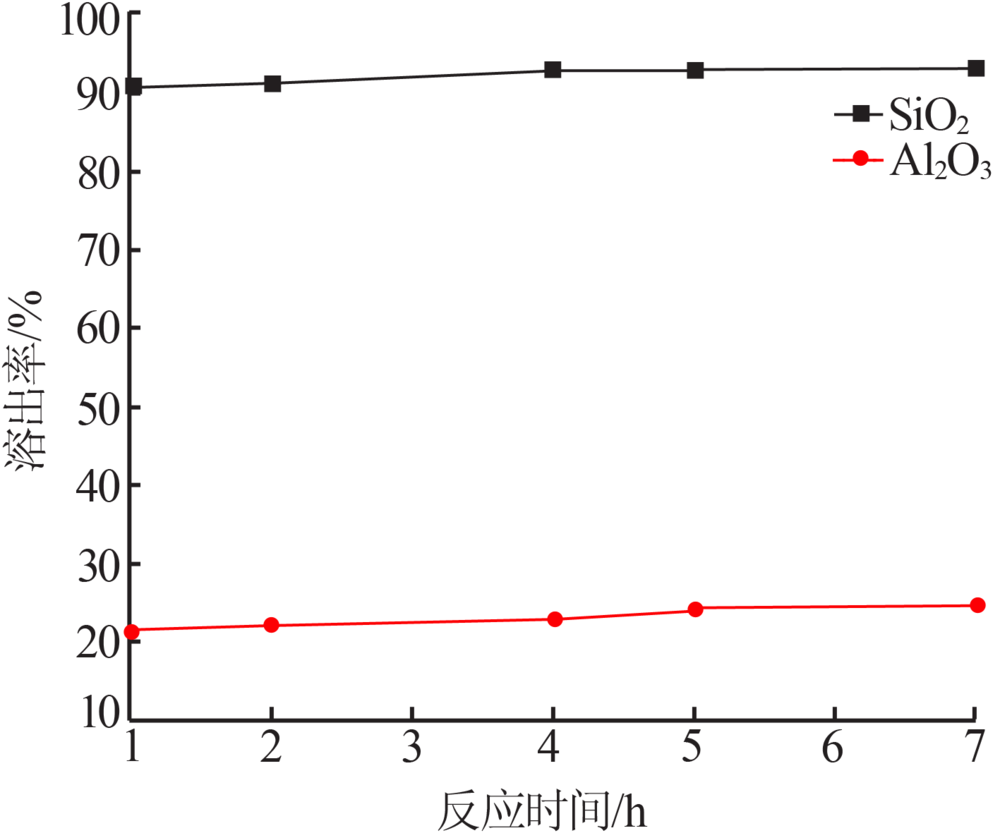
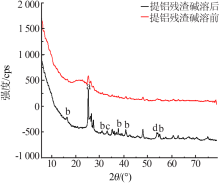
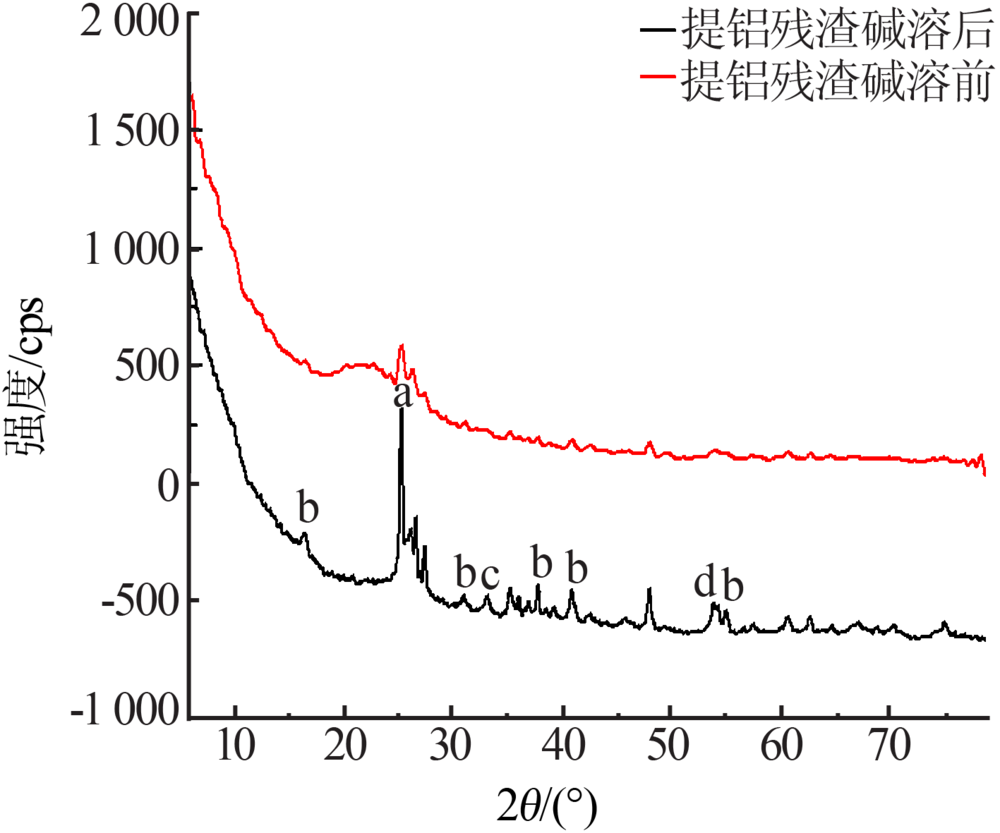
| [1] |
Blisserr R S, Rowson N A . A review of the multi-component utilization of coal fly ash[J]. Fuel, 2012,97:1-23.
doi: 10.1016/j.fuel.2012.03.024
|
| [2] |
刘晓婷, 王宝冬, 肖永丰 , 等. 高铝粉煤灰碱溶预脱硅过程研究[J]. 中国粉体技术, 2013,19(6):24-27.
|
| [3] |
Matjie R H, Bunt J R, van Heerden J H P , et al. Extraction of alumina from coal fly ash generated from a selected low rank bituminous South African coal[J]. Minerals Engineering, 2005,18:299-310.
|
| [4] |
李来时, 翟玉春, 秦晋国 , 等. 以粉煤灰为原料制备高纯氧化铝[J]. 化工学报, 2006,57(9):2189-2193.
|
| [5] |
曹君, 方莹, 范仁东 , 等. 粉煤灰提取氧化铝联产二氧化硅的研究进展[J]. 无机盐工业, 2015,47(8):10-13.
|
| [6] |
徐涛, 蓝海平, 杨超 , 等. 粉磨酸浸-氯化氢通气结晶法提取粉煤灰中氧化铝[J]. 无机盐工业, 2018,50(1):57-61.
|
| [7] |
曾丹林, 张琦, 刘胜兰 , 等. 粉煤灰中有价元素的回收利用研究[J]. 安徽农业科学, 2013,41(36):14006-14008.
|
| [8] |
Bengtson K B , A technological comparison of six processes for the production of reduction-grade alumina from non-bauxite raw materials[J]. Light Met., 1979, 217-282.
|
| [9] |
Livingston W R, Rogers D A, Chapman R J , et al. The effect of the calcination conditions on the leaching properties of the colliery spoil[J]. Hydrometallurgy, 1982,10:97-109.
|
| [10] |
王恩 . 煤粉炉粉煤灰与循环流化床粉煤灰矿物学性质比较[J]. 洁净煤技术, 2016(4):26-29.
|
| [11] |
郭昭华 . 粉煤灰“一步酸溶法”提取氧化铝工艺技术及工业化发展研究[J]. 煤炭工程, 2015,47(7):5-8.
|
| [12] |
李军旗, 浦锐, 陈朝轶 , 等. 碱法对粉煤灰的预脱硅处理[J]. 轻金属, 2010,11(8):11-13.
|