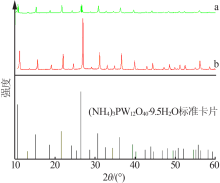
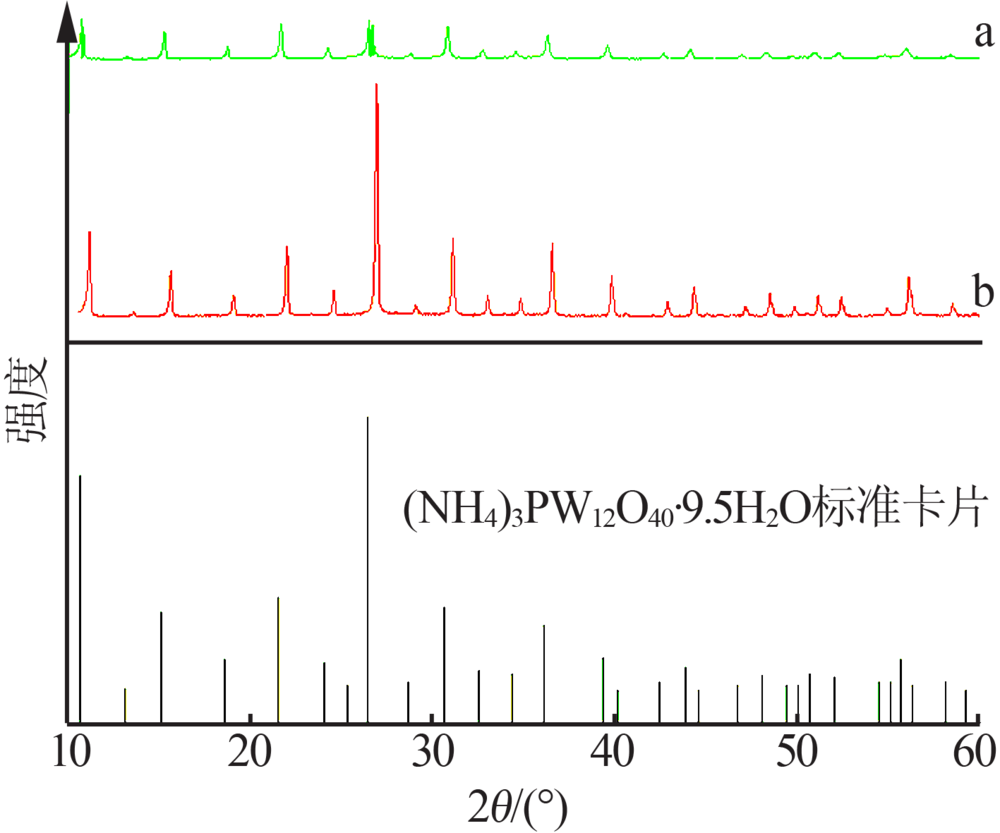
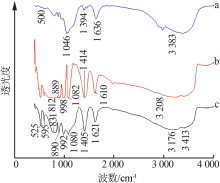
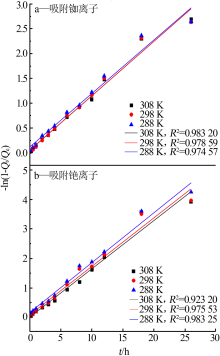
Inorganic Chemicals Industry ›› 2019, Vol. 51 ›› Issue (6): 34-37.
• Research & Development • Previous Articles Next Articles
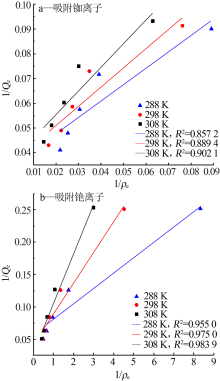
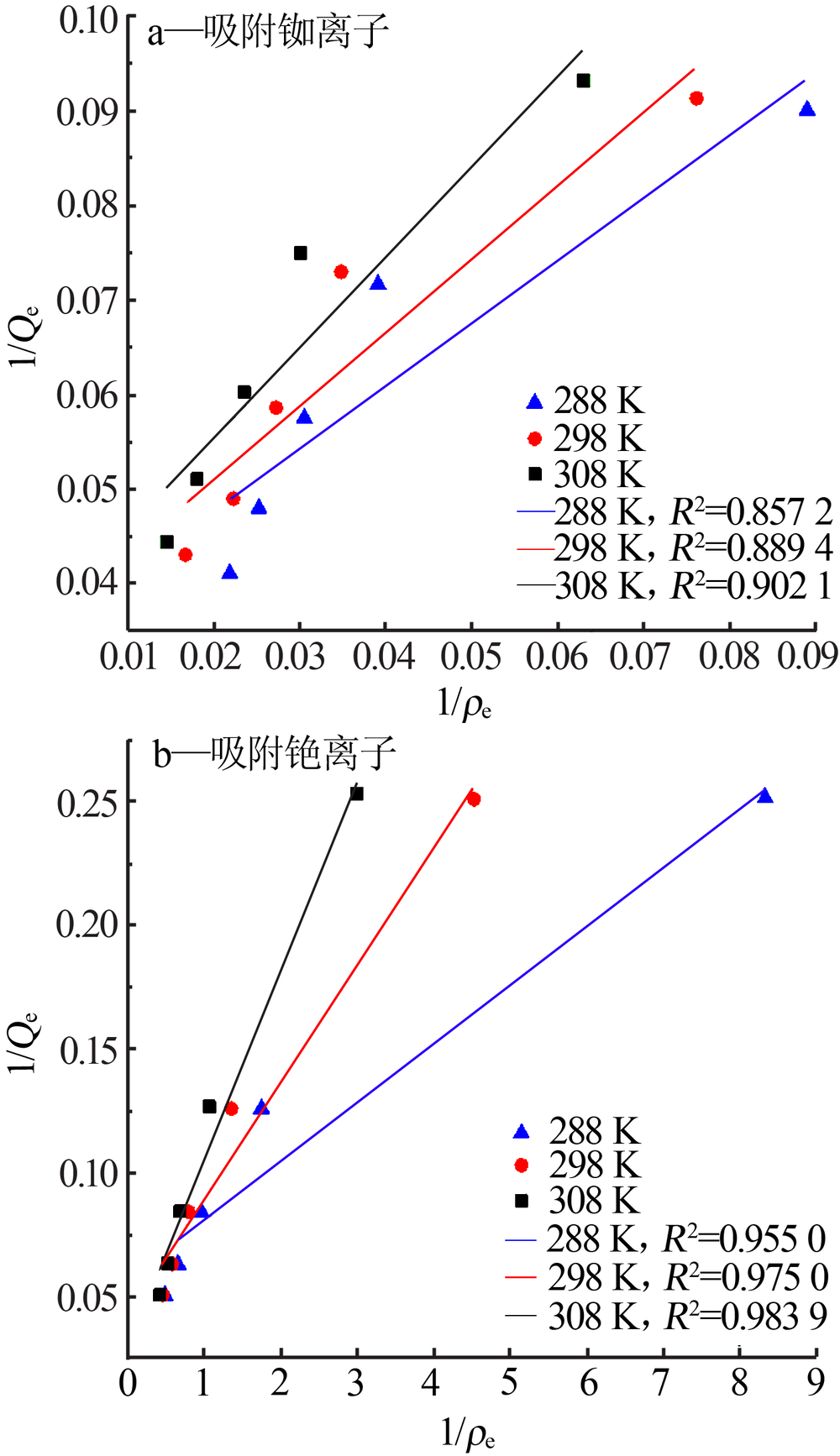
Study on adsorption properties of ammonium phosphate tungsten composites on rubidium and cesium
Li Ying1,2,Chen Xia1,2,Miao Shulan1,2,Liao Enxin1,2
- 1. College of Chemical Engineering and Materials Science,Tianjin University of Science and Technology,Tianjin,300457,China
2. Tianjin Key Laboratory of Marine Resources and Chemistry,Tianjin University of Science and Technology